Study population
Patients with ACLF Grades 1 and 2 presenting with ascites (an accumulation of fluid in the abdomen).

Current therapy
There are no specific therapies approved for patients with ACLF.

UNVEIL-IT ® Clinical study
UNVEIL-IT ® is a Phase 2 clinical study evaluating of the effects of VS-01 in patients with ACLF grades 1 and 2 with ascites.

What can you do?
Please speak with your healthcare team, family, and study staff to help decide if participating in the UNVEIL-IT ® study is right for you.

What is a clinical study ?
A clinical study (also known as a clinical trial) is designed to evaluate how safe and/or effective an investigational drug is in treating a specific condition. Before an investigational drug is made available to patients, regulatory authorities use the results of clinical studies to decide if an investigational drug is safe and effective and could be made available to more patients. Clinical studies are the only way we can develop new or better medical treatments and improve patient care.
Every clinical study is reviewed by an Independent Review Board/Ethics Committee that helps ensure that the study meets safety guidelines, and that the rights of participants are protected. Clinical studies are conducted by experienced and trained medical professionals who monitor the health of participants throughout the study.

What is ACLF ?
ACLF is a potentially life-threatening condition characterized by a group of signs that occur together (syndrome) in patients with late stage scarring or the liver (livercirrhosis).
ACLF is defined by organ dysfunctions and failures where an organ (liver, kidneys, brain, lungs,circulatory or blood clotting system) does not perform its expected function.
In patients with liver cirrhosis and acute hepatic decompensation, ACLF can be triggered by a precipitationg event(e.g., an infection) that leads to a progressive functional
deterioration of multiple organs with high short-term mortality (23% to 74% mortality at 28 days).
There are no specific therapies approved for patients with ACLF other than treating the triggering event such as, infection, and providing supportive care.
Liver transplantation remains the only cure, but is not an option to all patients in need.

What is the purpose of the UNVEIL-IT ® study ?
UNVEIL-IT ® is a study evaluating the efficacy, safety and tolerability of an investigational drug called VS-01 in patients with ACLF and ascites (a build-up of fluid in the abdomen). In this study, adult patients with ACLF will receive VS-01 on top of standard of care or standard of care alone
VS-01 is a test medicine not yet approved for market, which helps to remove ammonia and metabolites (substances produced by the body), and that are involved in the development and progression of ACLF.
In an earlier Phase 1b study VS-01 was administered for the first time to patients who presented with decompensated liver cirrhosis without ACLF. In this study, VS-01 was generally well tolerated and showed a favorable safety profile and promising preliminary efficacy results.
What happens in the UNVEIL-IT ® study ?
The study involves about 60 participants at research centers in Europe and the United States. The main part of the study lasts for approximately 1 week.
Participants will be followed until day 90.
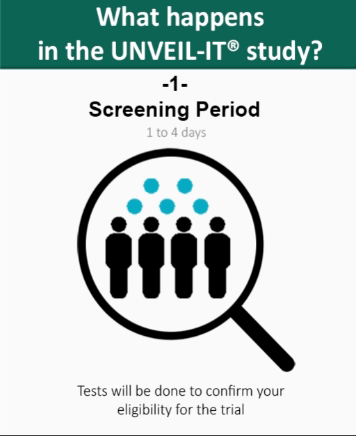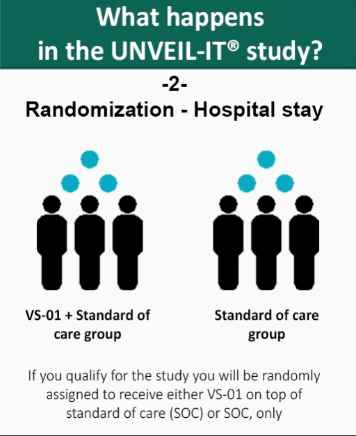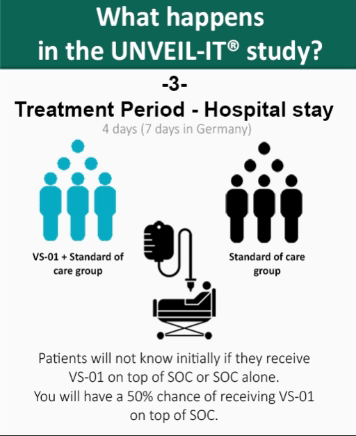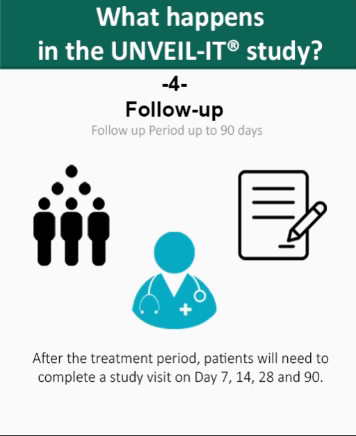
The screening period starts after the patient, or his/her legal representative provides consent to participate in the study and lasts for a maximum of 4 days (96 hours).
During this period, tests will be done to confirm patient’s
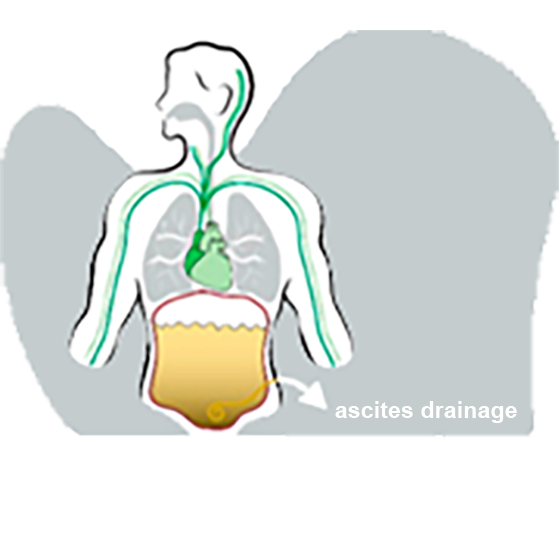
eligibility for the trial, including a full review of the patient’s medical history. During this period a catheter (tube) is inserted into the abdomen of the patient for removal of the fluid.
- Once patient’s eligibility is confirmed, the patient is randomly assigned to receive either VS-01 and standard of care, or standard of care alone.
- After randomization, the patient receives VS-01 and standard of care, or standard of care alone for a period of 4 days (hospital stay). Providing the patient’s medical condition does not require further hospitalization, patient’s discharge may happen as early as Day 5 (Day 7 in Germany).
- After discharge, the patient is asked to return on Days 7, 14, 28 and 90 for a study visit where the investigator performs tests and checks the patient’s medical condition. The follow up period is up to 90 days.
What is VS-01 ?
VS-01 is an investigational drug that is administered intraperitoneally (into the abdominal cavity) and left to dwell for 3 hours. VS-01 comes in a liquid form containing free citric acid and lipid droplets made of fatty substances that
may naturally occur in human bodies (liposomes) and which also include citric acid.
The liposomes and surrounding fluid scavenge ammonia and other metabolites. At the end of the dwell time, VS-01, loaded with ammonia and other metabolites, is drained from the abdominal cavity.
There by these toxic substances are removed from the body.
VS-01 aims to support the liver, brain and kidney function, the three organs that primarily fail and substantially worsen the prognosis of patients with ACLF.
It is not approved by any regulatory authority.
Who can participate in the UNVEIL-IT ® study ?
Patients are eligible to participate if they :
- are 18 to 79 years of age (men and women)
- do have ACLF grades 1 or 2 on the background of liver cirrhosis*
- do have an onset of ACLF not more than 7 days (168 hours) before baseline
- do have ascites requiring paracentesis (removal of fluid through a needle)
Other criteria will be reviewed to determine if you may participate in the study.
*according to EASL-CLIF criteria, please visit : https://efclif.com/research-infrastructure/score-calculators/clif-c-of-aclf-ad/ for further informations
Why participate in a clinical study ?
There are several reasons to participate in the study:
- Study participants will receive study-related care from specialized medical professionals
- Study participants will learn more about their disease and your health
- Patient participation may help advance the research of treatments for ACLF
Not everyone enrolled in the study will benefit directly. The health of study participants may improve or may not improve at all.
It is also possible that some participants may feel worse. Taking part in a clinical study is completely voluntary.
If enrolled, you can choose to end their participation at any time and for any reason without any penalty.